CUET UG CHEMISTRY EXAM PAPER 2024
CUET Chemistry Exam Paper 2024
1. The total number of ions produced from the complex [Cr(NH3)6]Cl3 in aqueous solution will be _____
(1) 2
(2) 3
(3) 4
(4) 5
Solution :
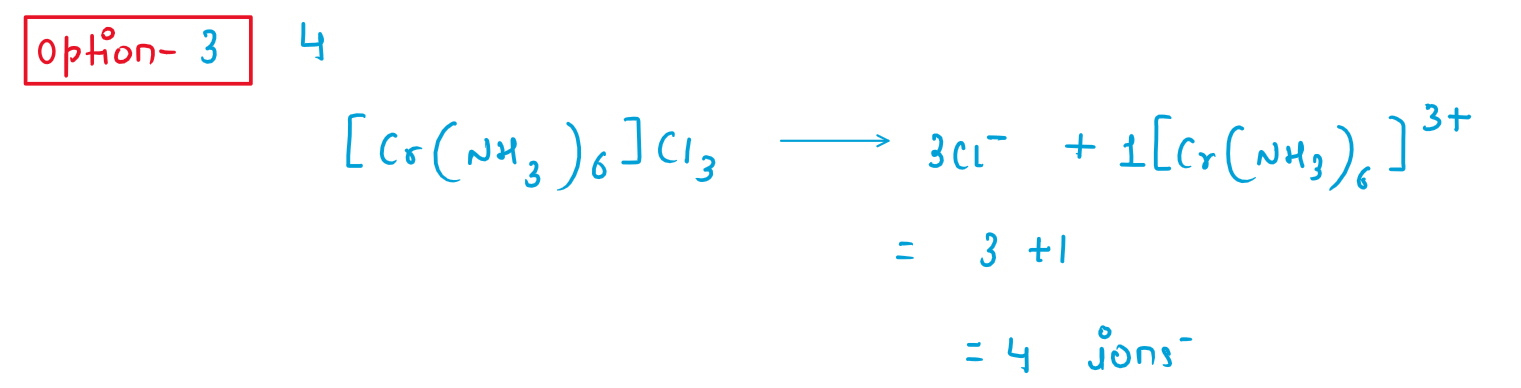
2. Arrange the following in decreasing order of number of molecules contained in:
(A) 16g of O2
(B) 16g of CO2
(C) 16g of CO
(D) 16g of H2
Choose the correct order from the options given below:
(1) (A), (B), (C), (D)
(2) (D), (C), (A), (B)
(3) (B),(A),(D),(C)
(4) (C),(B),(D),(A)
Solution
3. A molecule X associates in a given solvent as per the following equation:
X⇋(X)n
For a given concentration of X,the van't Hoff factor was found to be 0.80 and the fraction of associate molecules was 0.3.The correct value of 'n' is :
(1) 2
(2) 3
(3) 1
(4) 5
Solution
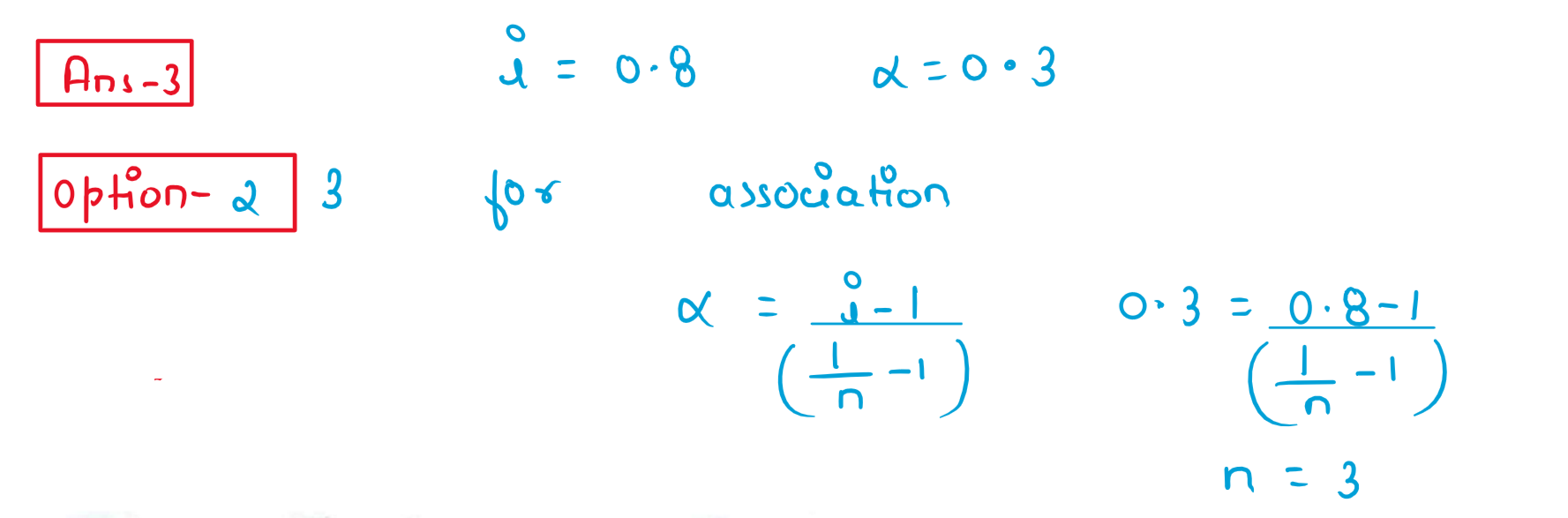
4. the oxidation number of Co in complex [Co(H2NCH2CH2NH2)3]2(SO4)3 is:
(1) 3
(2) 4
(3) 2
(4) 5
Solution
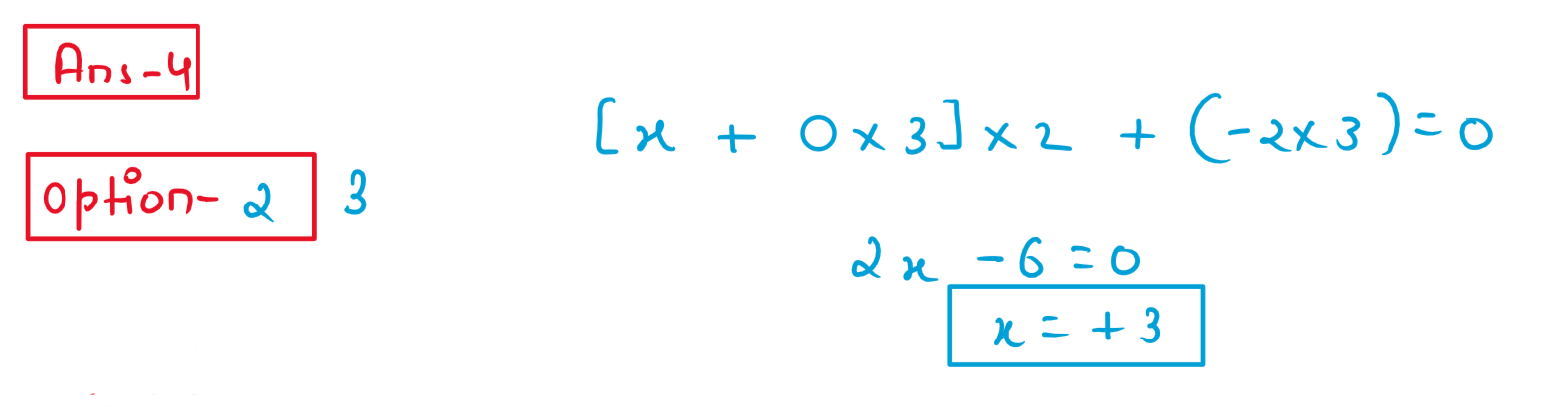
5. The correct structure of dipeptide,Gly-Ala(glycyl alamine) is
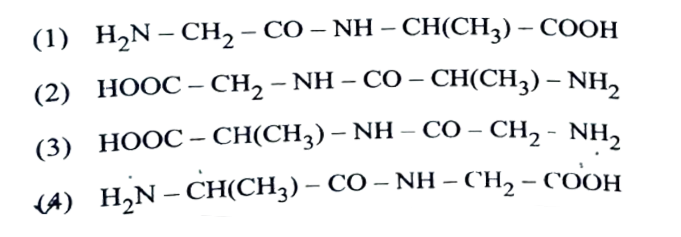 Solution
Solution
6. The Cu metal crystallises into fcc lattice with a unit cell edge length of 361pm.The radius of Cu atom is:
(1) 127pm
(2) 181pm
(3) 157pm
(4) 108pm
Solution
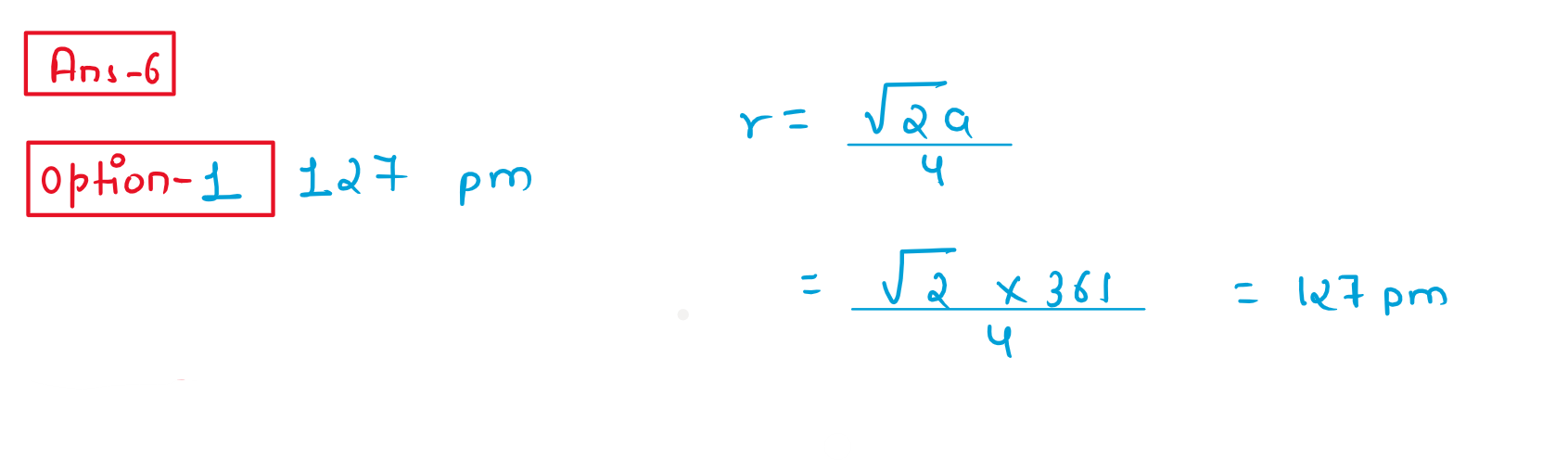
7. If 75% of a first order reaction gets completed in 32minutes,time taken for 50% completion of this reaction is:
(1) 16minutes
(2) 78minutes
(3) 8minutes
(4) 4 minutes
Solution
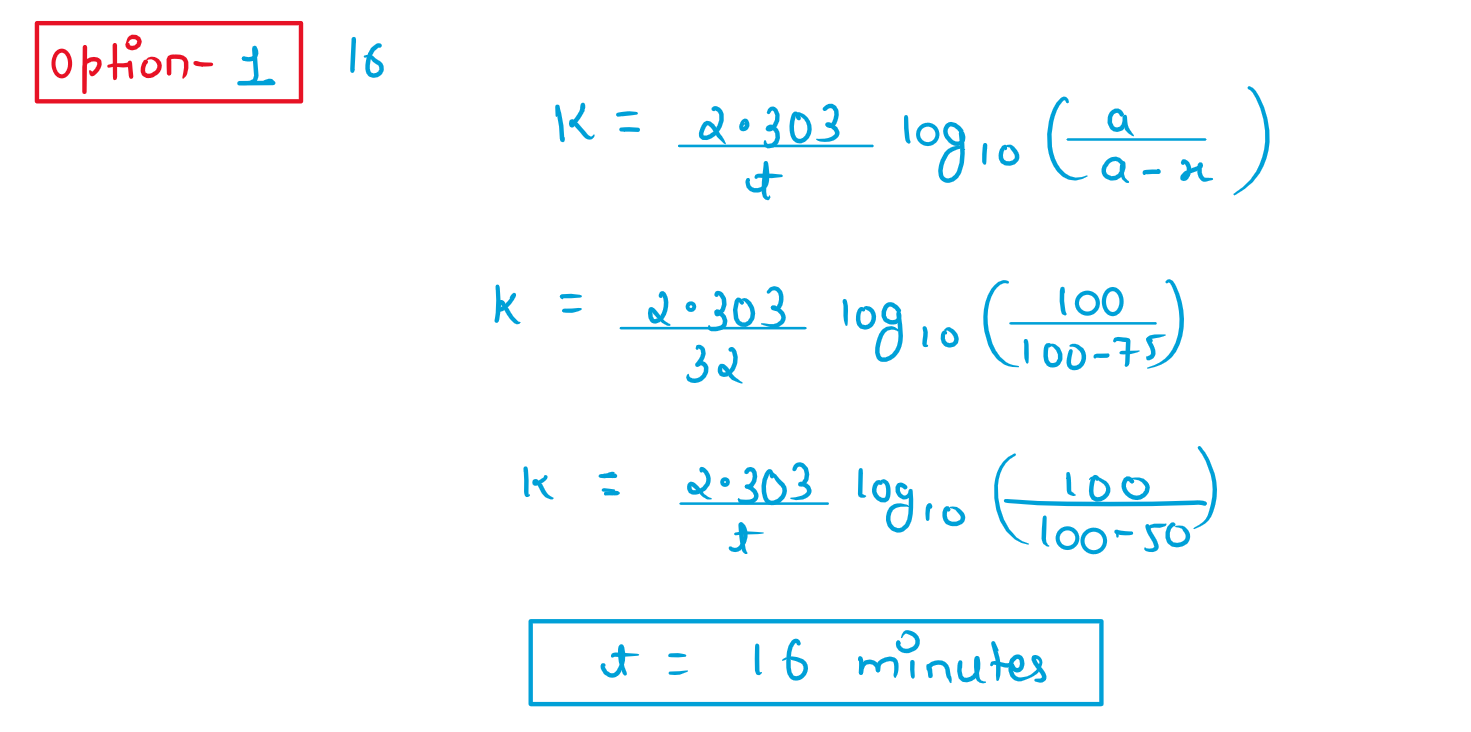
8. Which of the following compounds will be repelled when placed in an external magnetic field?
(1) Na2[CuCl4]
(2) Na2[CdCl4]
(3) K4[Fe(CN)6]
(4) K3[Fe(CN)6]
Solution
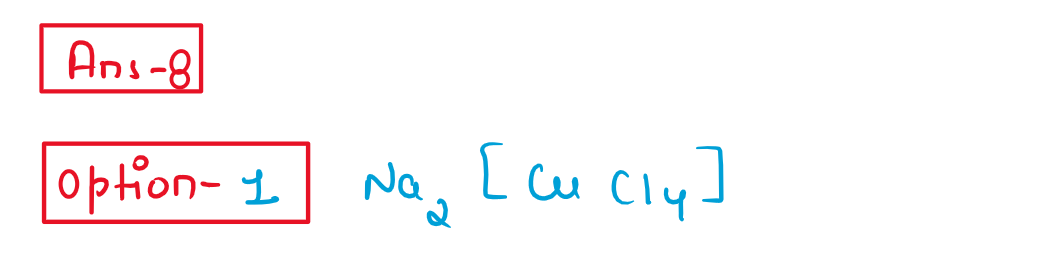
9. The spin only magnetic moment of Hexacyanidomaganate(II) ion is _____ BM
(1) 5.90
(2) 1.73
(3) 4.90
(4) 3.87
Solution
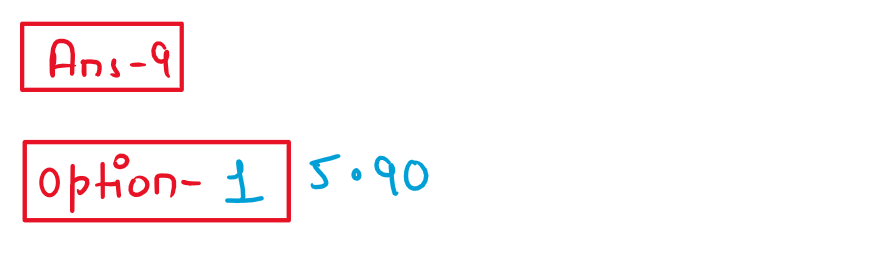
10. The correct order of increasing boiling points of the following compounds is :
Pentan-1-ol, n-Butane, Pentanal, Ethoxyethane
(1) Ethoxyethane, Pentanal, n-Butane, Pentan-1-ol
(2) Pentanal, n-Butane, Ethoxyethane, Pentan-1-ol
(3) n-Butane, Pentanal, Ethoxyethane, Pentan-1-ol
(4) n-Butane, Ethoxyethane, Pentanal, Pentan-1-ol
Solution

11. In the following reaction,identify the product D.
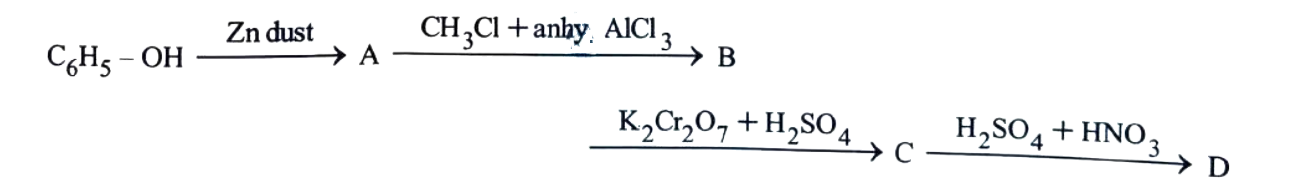
(1) o-Nitrobenzoic acid
(2) p-Nitrobenzoic acid
(3) o,p-Dinitrobenzoic acid
(4) m-Nitrobenzoic acid
Solution
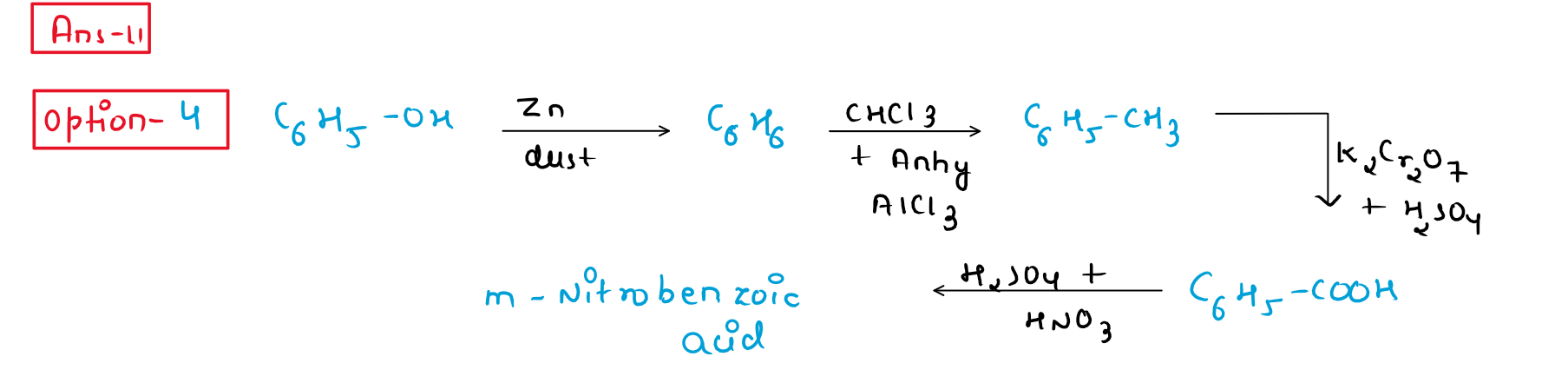
12. The gold number range of some of the lyophilic colloids is given below:
A:0.005-0.01,B:0.15-0.25,C:0.04-1.0 and D:15-25.
Which among these can be used as a better pro active colloid
(1) A
(2) B
(3) C
(4) D
Solution
13. Reaction of aniline with conc. HNO3 and conc. H2SO4 at 298K will produce 47% of
(1) p-Nitroaniline
(2) o-Nitroaniline
(3) m-Nitroaniline
(4) 2,4-Dinitroaniline
Solution
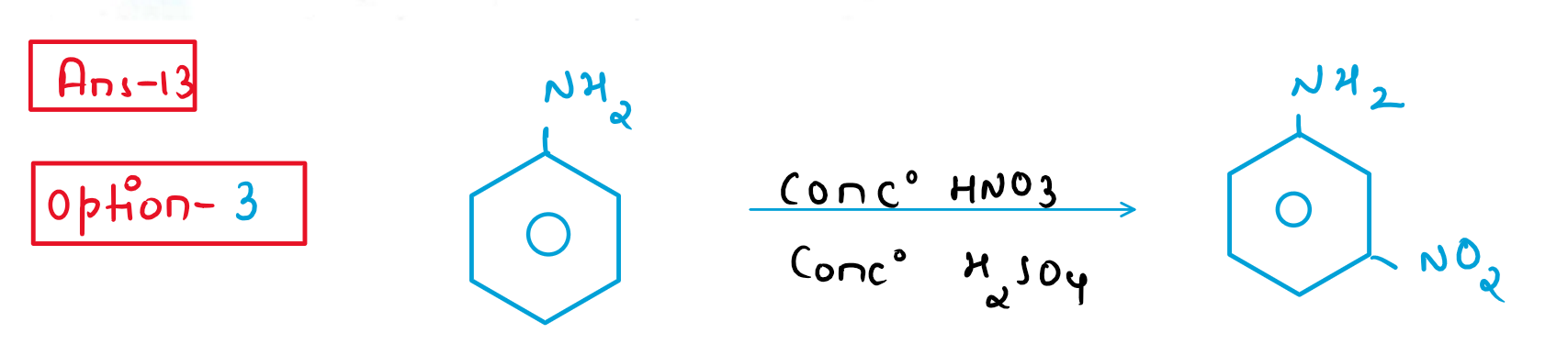
14. What will be increasing order of basic strength of the following compounds ?
C2H5NH2, (C2H5)2NH,(C2H5)3N,C6H5NH2
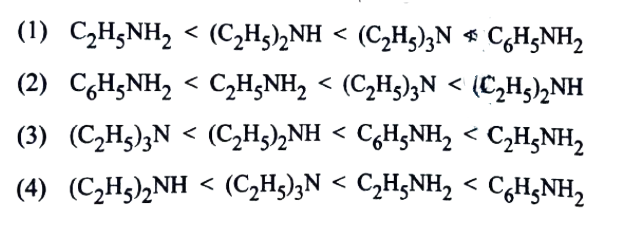 Solution
Solution

15. Which of the following compounds will give Hell-Volhard-Zelinsky reaction
(1) R-CH2-COOH
(2) R3C-CHO
(3) R2CO
(4) H-COOH
Solution
16. Arrange the following acids in increasing order of their acidic strengths:
HCOOOH,FCH2COOH,NO2CH2COOH
,CICH2COOH
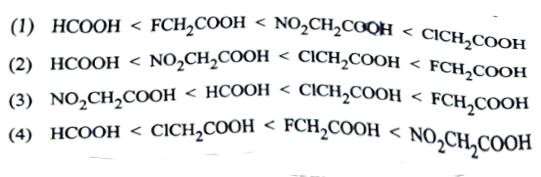 Solution
Solution
17. In the following compounds,what is the 1ncreas1ng order of their reactivity towards nucleophilic addition reactions?
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone
(1) Benzaldehyde < p-Tolualdehyde < p-Nitrobenzaldehyde < Acetophenone
(2) Acetophenone < Benzaldehyde < p-Tolualdehyde < p-Nitrobenzaldehyde
(3) Acetophenone < p-Tolualdehyde < Benzaldehyde < p-Nitrobenzaldehyde
(4) Benzaldehyde < Acetophenone < p-Tolualdehyde < p-Nitrobenzaldehyde
Solution

18. The Gatterman-Koch reaction is used in the industrial preparation o fbenzaldehyde. The electrophile
involved in this reaction is
(1) CO+
(2) HCl+CO2+anhydrous AlCl3
(3) HCO+
(4) CO+anhydrous AlCl3
Solution
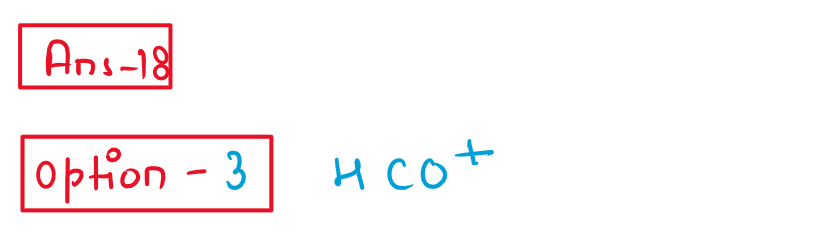
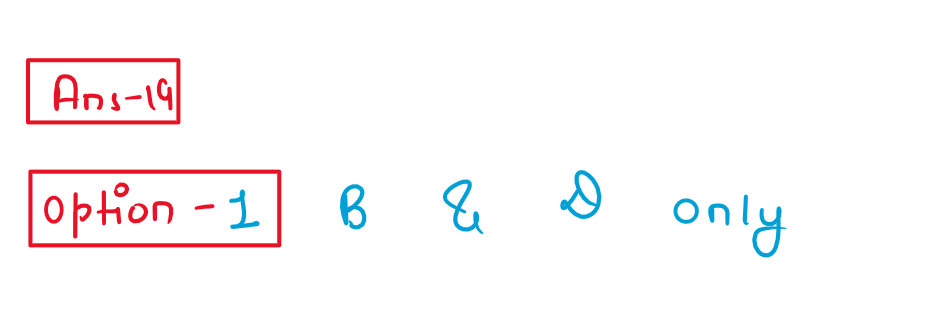
19. Formaldehyde undergoes Cannizzaro reaction because
(A) It has alpha-hydrogen atom.
(B) It does not have alpha-hydrogen atom.
(C) It does not undergo self-oxidation and reduction on heating with concentrated alkali.
(D) It undergo self-oxidation and reduction on heating with concentrated alkali.
Choose the correct answer from the options given below:
(1) (B) and (D) only
(2) (A) and (C) only
(3) (B) and (C) only
(4) (A) and (D) only
Solution
20. In the reaction, (CH3)3C-O-CH3+HI -> Products
CH3OH and (CH3)3Cl are the products and not CH3I and (CH3)3C-OH.It is because
(A) in step 2 of the reaction the departure of leaving group (HO - CH3) creates less stable carbocation.
(B) in step 2 of the reaction the departure of leaving group (HO - CH3) creates more stable carbocation.
(C) the reaction follows SNI mechanism.
(D) the reaction follows SN2 mechanism
Choose the correct answer from the options given below :
(1) (B) and (D) only
(2) (B) and (C) only
(3) (A) and (D) only
(4) (A) and (C) only
Solution
21. Aniline does not undergo Friedel-Crafts reaction because
(A) It forms salt with the Lewis acid catalyst, AlCl3
(B) Nitrogen of aniline acquires negative charge.
(C) Nitrogen of aniline acquires positive charge.
(D) Nitrogen acts as a strong deactivating group in the further reaction.
Choose the correct answer from the options given below:
(1) (A), (B) and (D) only
(2) (A), (B) and (C) only
(3) (A), (C) and (D) only
(4) (B), (C) and (D) only
Solution
22. Although chlorine is an electron withdrawing ~~up, yet it is ortho- and para-directing in electrophilic
aromatic substitution reaction because
(A) Chlorine withdraws electrons through inductive effect.
(B) Chlorine destabilises the intermediate carbocation formed during electrophilic substitution.
(C) Chlorine accepts electrons through resonance.
(D) Chlorine releases electrons through resonance
Choose the correct answer from the options given below :
(1) (A), (B) and (D) only
(2) (A), (B) and (C) only
(3) (A), (C) and (D) only
(4) (B), (C) and (D) only
Solution
23. In Etard reaction, the final product is:
(1) Aromatic aldehyde
(2) Aromatic chloride
(3) Aromatic amine
(4) Aromatic alcohol
Solution
24. Match List-I with List-II:
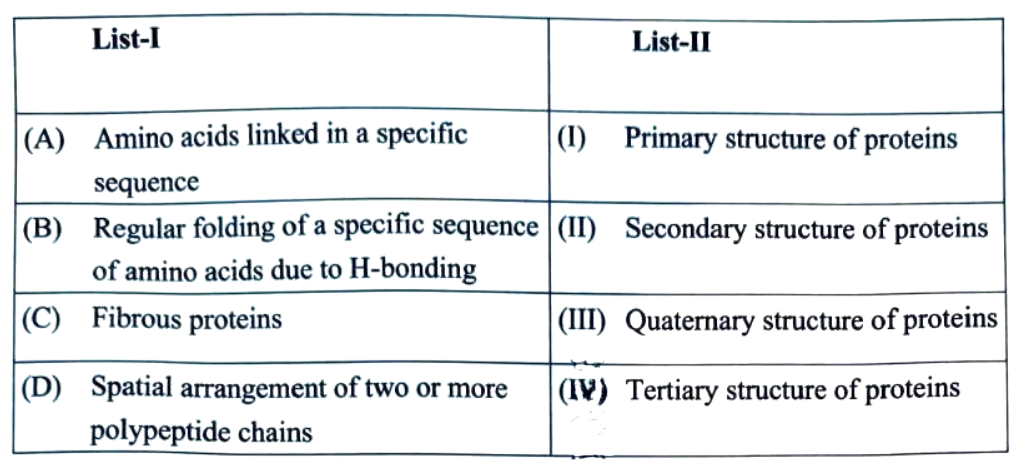
Choose the correct answer from the options given below :
(1) (A)-(I),(B)-(II),(C)-(III),(D)-(IV)
(2) (A)-(I),(B)-(III),(C)-(II),(D)-(IV)
(3) (A)-(I),(B)-(II),(C)-(IV),(D)-(III)
(4) (A)-(III),(B)-(IV),(C)-(I),(D)-(II)
Solution

25. Match List-I with List-II:

Choose the correct answer from·the options given below :
(1) (A) - (III), (B) - (IV), (C) - (II), (D) - (I),
(2) (A) - (IV), (8) - (III), (C) - (I), (D) - (II)
(3) (A) - (I), (B) - (IV), (C) - (II), (D) - (III)
(4) (A) - (III), (B) - (I), (C) - (IV), (D) - (II)
Solution

26. Match List-I with List-II:

Choose the correct answer from·the options given below :
(1) (A) - (I), (B) - (II), ( C) - (III), (D) - (IV)
(2) (A) - (I), (B) - (III), (C) - (II); (D) - (IV)
(3) (A) - (I), (B) - (II), (C) - (IV), (D) - (III)
(4) (A) - (III), (B) - (IV), (C) - (I), (D) - (II)
Solution

27. Match List-I with List-II:

Choose the correct answer from·the options given below :
(1) (A) - (III), (B) - (II), (C) - (I), (D) - (IV)
(2) (A) - (II), (B) - (III), (C) - (I); (D) - (IV)
(3) (A) - (III), (B) - (II), (C) - (IV), (D) - (I)
(4) (A) - (II), (B) - (III), (C) - (IV), (D) - (I)
Solution

28. In tbe following table, match the reactants given in List-I with the correct product in List-II as per
the reaction of hydration of alkene under acidic condition

Choose the correct answer from the options given below:
(1) (A) - (1), (B) - (II), (C) - (III), (D) - (IV)
(2) (A) - (1), (B) - (III), (C) - (II), (D) - (IV)
(3) (A) - (II), (B) - (I), (C) - (IV), (D) - (III)
(4) (A) - (Ill), (B) - (IV), (C) - (I), (D) - (II)
Solution

29. Which among the following is not an Analgesic ?
(1) Morphene
(2)Heroin
(3)Codeine
(4)Ranitidine
Solution

30. The increasing order of acidity of the following compounds based on pKa values is
BrCH2COOH
ClCH2COOH
FCf2COOH
HCOOH
Choose the correct answer from the options given below :
(1) (D) <(A)< (B) < (C)
(2) (A)< (D) < (C) < (B)
(3) (B) <(A)< (D) < (C)
(4) (C) < (B) < (D) < (A)
Solution
31. For SN2 Reaction, the increasing order of the reactivity of the following allkyl halides is
(1) CH3CH2CH2CH2Br
(2) CH3CH2CH(Br)CH3
(3) (CH3)3CBr
(4) (CH3)2CHCH2Br
Choose the correct answer from the options given below :
(1) (A) <(B)< (C) < (D)
(2) (A)< (C) < (B) < (D)
(3) (B) <(A)< (D) < (C)
(4) (C) < (B) < (D) < (A)
Solution
32. Read the following passage and answer the next five questions based on it.
Battery or cell converts chemical energy of the redox reaction to electrical energy. In fuel cell (a galvain cell), the chemical energy of combustion of fugle like H2 ethanol, etc. are directly converted to electrical energy. In a fuel cell, H2 and O2 react to produce electricity, where H2 gas is oxidised at anode and oxygen is reduced at cathode and the reactions involved are
Anode reaction: : H2 + 2 +2OH_ ------ 2H2O +2e-
Cathode reaction: O 2 +2H2O+ 4e_ ------4OH-
67.2 L of H2 at STP reacts in 15 minutes.
32. The number of moles of hydrogen oxidised is:
(1) 0.33 moles
(2) 33.3 moles
(3) 3.0 moles
(4) 1.33 moles
Solution

33. Read the following passage and answer the next five questions based on it.
Battery or cell converts chemical energy of the redox reaction to electrical energy. In fuel cell (a galvain cell), the chemical energy of combustion of fugle like H2 ethanol, etc. are directly converted to electrical energy. In a fuel cell, H2 and O2 react to produce electricity, where H2 gas is oxidised at anode and oxygen is reduced at cathode and the reactions involved are
Anode reaction: : H2 + 2 +2OH_ ------ 2H2O +2e-
Cathode reaction: O 2 +2H2O+ 4e_ ------4OH-
67.2 L of H2 at STP reacts in 15 minutes.
The number of moles of electricity produced in the oxidation of 67.2 L. of H2 at STP in:
(1) 2 moles
(2) 4 moles
(3) 1 mole
(4)6 moles
Solution
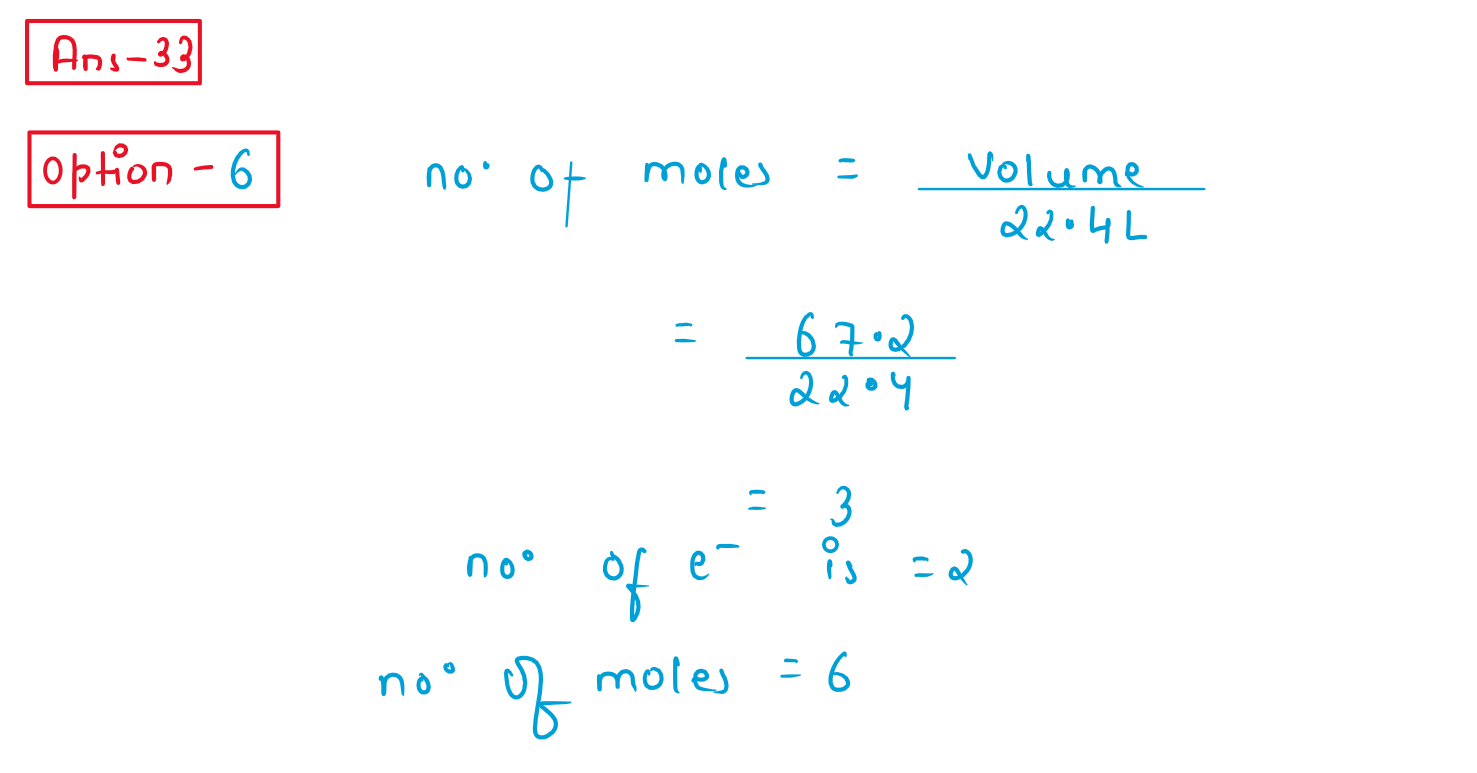
34. Read the following passage and answer the next five questions based on it.
Battery or cell converts chemical energy of the redox reaction to electrical energy. In fuel cell (a galvain cell), the chemical energy of combustion of fugle like H2 ethanol, etc. are directly converted to electrical energy. In a fuel cell, H2 and O2 react to produce electricity, where H2 gas is oxidised at anode and oxygen is reduced at cathode and the reactions involved are
Anode reaction: : H2 + 2 +2OH_ ------ 2H2O +2e-
Cathode reaction: O 2 +2H2O+ 4e_ ------4OH-
67.2 L of H2 at STP reacts in 15 minutes.
The quantity of electricity produced in the oxidation of 67.2 L. of H2 at STP in: at STP in: be
(1)96500 C
(2) 579000 C
(3)193000 C
(4)48250 C
Solution
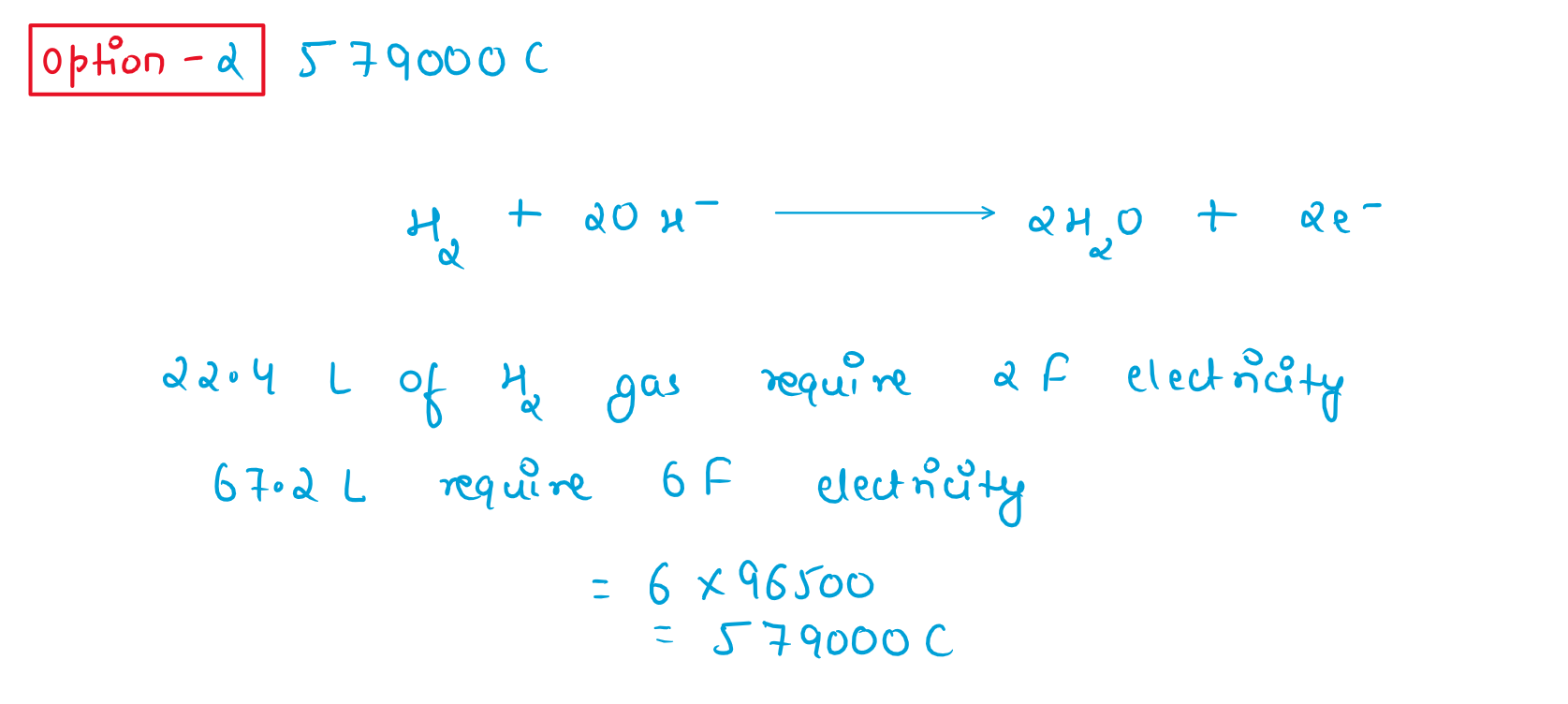
35. Read the following passage and answer the next five questions based on it.
Battery or cell converts chemical energy of the redox reaction to electrical energy. In fuel cell (a galvain cell), the chemical energy of combustion of fugle like H2 ethanol, etc. are directly converted to electrical energy. In a fuel cell, H2 and O2 react to produce electricity, where H2 gas is oxidised at anode and oxygen is reduced at cathode and the reactions involved are
Anode reaction: : H2 + 2 +2OH_ ------ 2H2O +2e-
Cathode reaction: O 2 +2H2O+ 4e_ ------4OH-
67.2 L of H2 at STP reacts in 15 minutes.
If the entire current produced is used for the electrodeposition of Silver (at. wt. 108 g mol-1) from Silver (1) the 35. solution, amount of deposited will be
(1)324 g
(2) 648 g
(3)108g
(4)216 g
Solution
36. The source of electrical energy on the Apollo moon flight was :
(1) Lead storage battery
(2) A generator se
(3) Ni-Cd cells
(4)H2-O2 Fuel cell
Solution
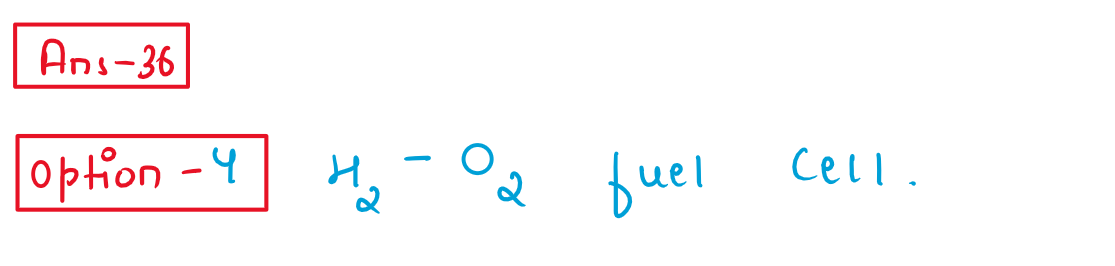
Read the following passage and answer the next five questions based on it.
Sc Ti V Cr Mn Fe Co Ni Cu Zn Y Zr Nb Mo Tc Ru Rh Pd Ag Cd La Hf Ta W Re Os Ir Pt Au Hg
In any transition series, as we move from left to right the d-orbitals are progressively filled and their properties vary accordingly.
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
The above are the two series of f-block elementh which the chemical properties won't change mach. The
5f-series elements are radioactive in nature andrijostly are artificially synthesized in laboratories and thus much is not known about their chemical properties.
37.Identify the incorrect statement.
(1) Second ionisation enthalpy of Ag is greater than second ionisation enthalpy of Pd.
(2) Zr and Hf shares almost identical nuclear properties.-
(3) Melting point of Mn is lower than that of C90
(4)Interstitial compounds are non-stoichiomet et and neither ionic nor covalent in nature
Solution
38.Which of the following is the correct order of sefond ionisation enthalpy?
(1) V>Cr>Mn
(2)V< Cr < Mn
(3)V < Cr > Mn
(4)V > Cr < Mn
Solution
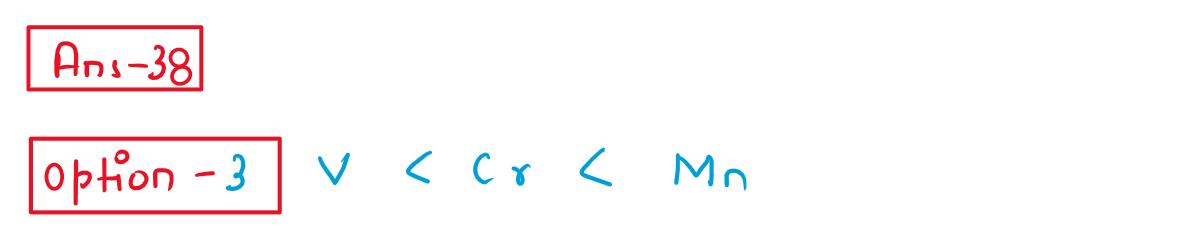
39. Which of the following pair of compounds exhil same colour in aqueous solution?
(1) FeCl2, CuCl2
(2)VOCI2 CuCl2
(3)VOCI2,FeCl2
(4) VOCI2, MnCl2
Solution
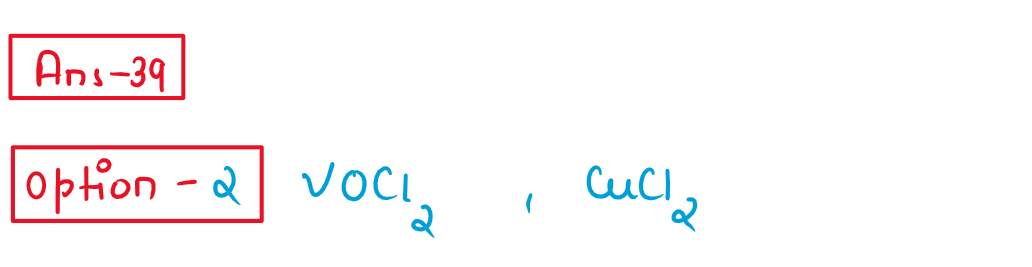
40. Which metal has the highest
oxidation state in the first row transition series?
(1) Cr.
(2) Fe
(3)Mn
(4) V
Solution

41. Why do the actinoids eshihit higher number of dation states than lanthanoids?
(1)4f orbitals are more diffused than the Sf orbitals
(2) Energy difference between 5f and 6d is les with respect to the energy difference between 4f and 5d.
(3) Energy difference between 5f and fid is man with respect to the energy difference between 4f and 5d
(4) Actinoids are more reactive in nature than the lanthatnoids.
Solution

42. Camphor in nitrogen gas a type of solution
(1)Gas-Gas
(2) Solid - Gas
(3) liquid-Gas
(4) Solid- Liquid.
Solution
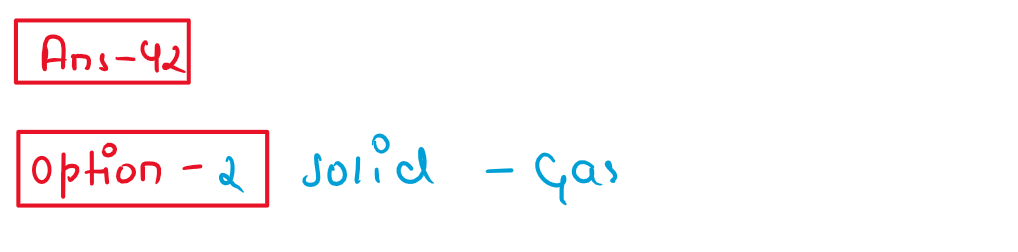
43. Identify the correct order of organic compounds in the following chenical reaction:

(1)CH3MgBr
(2)CH3Br
(3)CH3cl
(4)CH4
Choose the correct answer from the options given below :
(1)(B), (A), (D), (C)
(2)(A), (C), (B), (D)
(3)(B), (A), (C), (D)
(4)(C), (B), (D), (A)
Solution
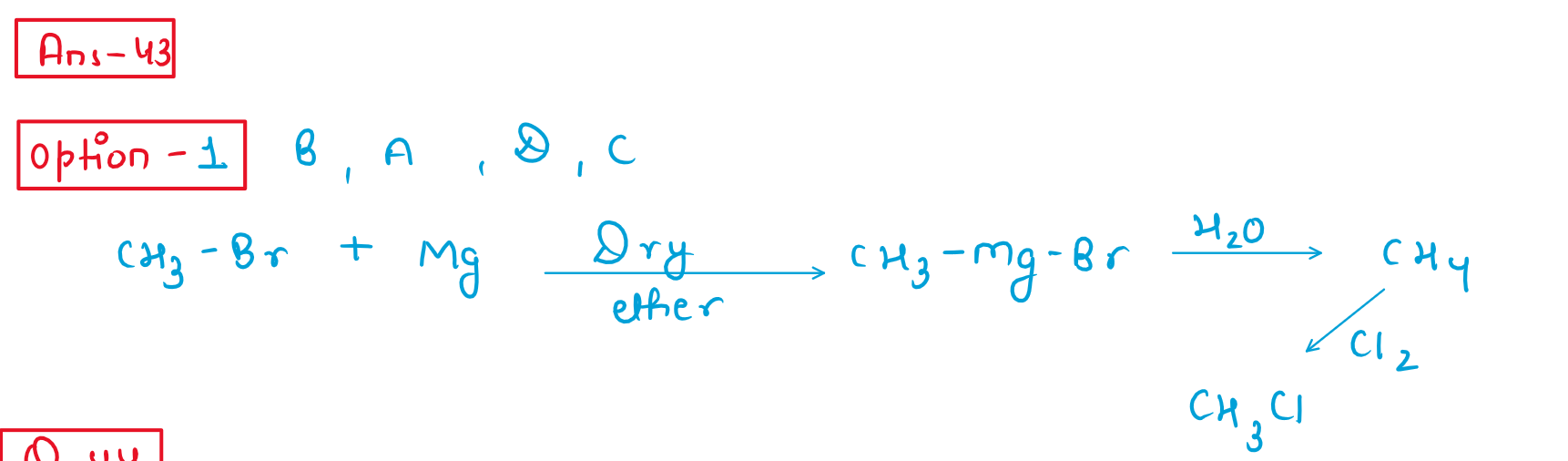
44. Consider the following statements regarding osmotic pressure :
(1)Molar mass of a protein can be determined using osmotic pressure method.
(2)The osmotic pressure is proportional to the mola.
(3) Reverse osmosis occurs ,when a·pressure larger than osmotic pressure is applied to the concentrated
solution side.
(4) Edema occurs due to retention of water in tissue cells as a result of osmosis.
Choose the correct statements with reference to osmotic pressure :
(1) (A), (B) and (D) only
(2)(A), (B) and (C) only
(3) (A), (B), (C) and (D)
(4) (B), (C) and (D) only
Solution

45. Vapour pressures of pure liquids 'A' and 'D at 50C are 500mm Hg and 800 mm Hg respectively The binary soluction of 'A' AND 'D' boils at 50C and 700mm Hg pressure the mole perecentage of 'D' in the soluction is:
(1) 33.33 mole percent
(2) 66.67 mole percent
(3) 25.75 mole percent
(4) 75.25 mole percent
Solution
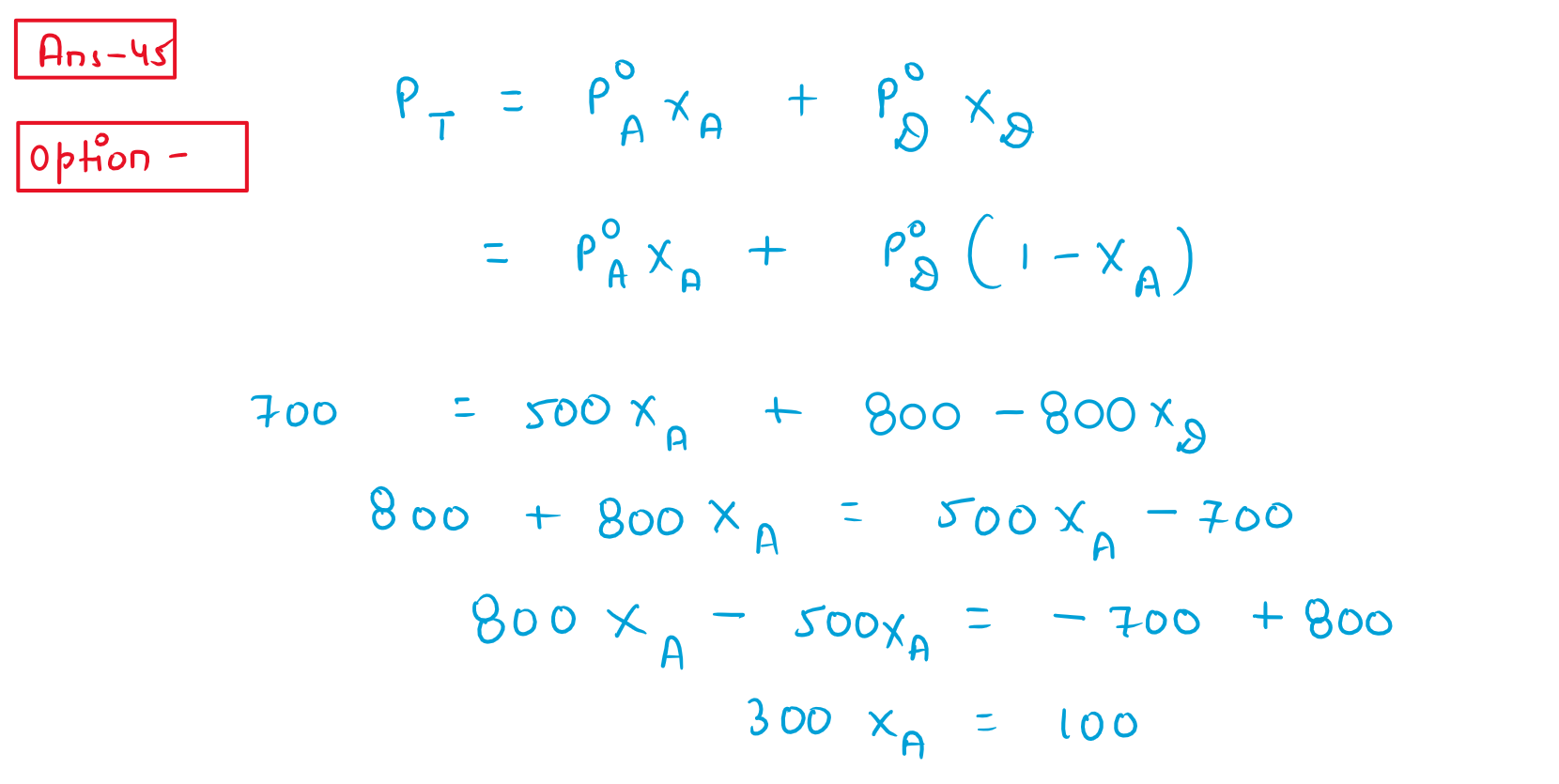
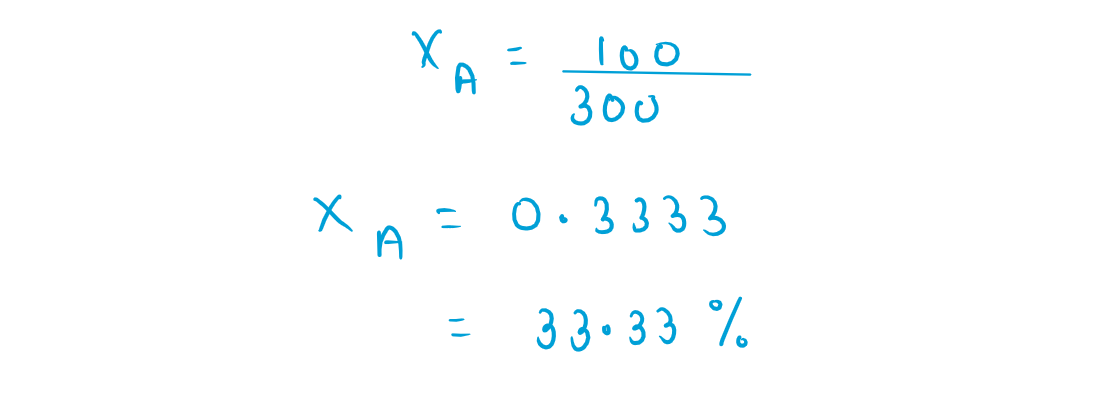
46. For the following reaction :

volume is increased to double its value by decreasing the pressure it If the reaction·is first order with respect to X and second order with respect to A2the rate of reaction will
(1) Decrease by eight times of its initial value
(2) Increase by eight times of its initial value
(3) Increase by four times of its initial value
(4)Remain unchanged
Solution
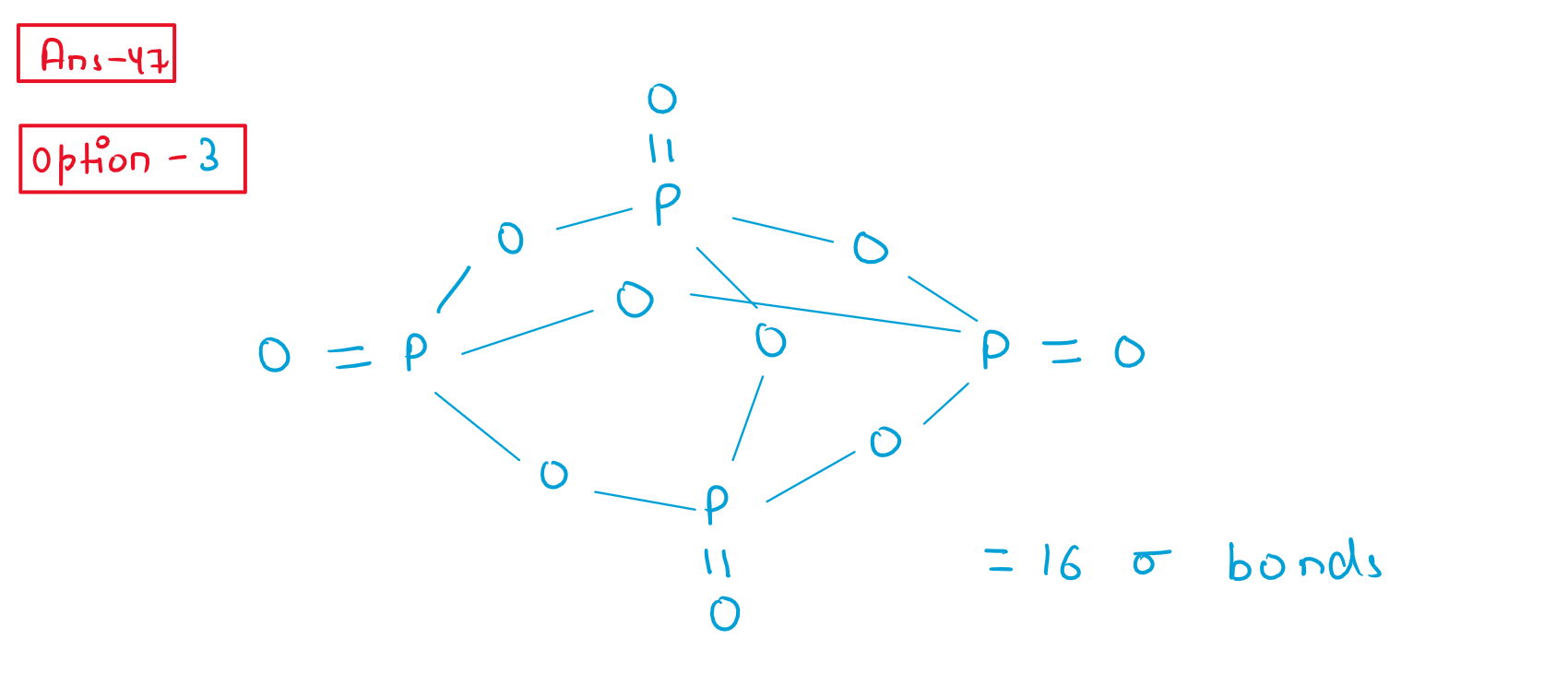
47. The total number of sigma bonds present in o10are :
(1) 6
(2) 7
(3) 16
(4) 17
Solution
48. In the electrolysis of alumina to obtain Alumimium metal, the cryolite is added mainly to
(1) lower the melting point of alumina.
(2) dissolve the alumina in the molten cryolite.
(3) remove the impurities of alumina.
(4) increase the electrical conductivity.
Solution

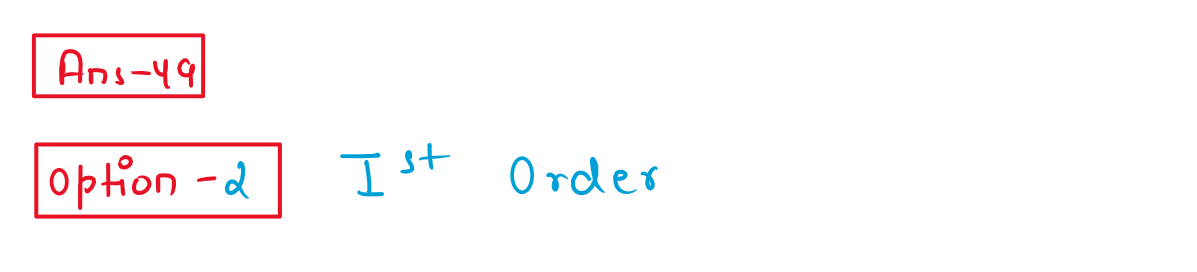
49. Identify the order of reaction if its rate constant is k = 2 x 10-2 s-1
(1) Zero order
(2)First order
(3) Second order
(4) Half order
Solution
50. For a complex reaction, the order of reaction is equal to
(1) Sum of stoichiometric coefficients in balanced chemical reactiqn
(2)The molecularity of overall reaction
(3) Order of fastest step of the reaction
(4) The molecularity of slowest step of Reaction
Solution
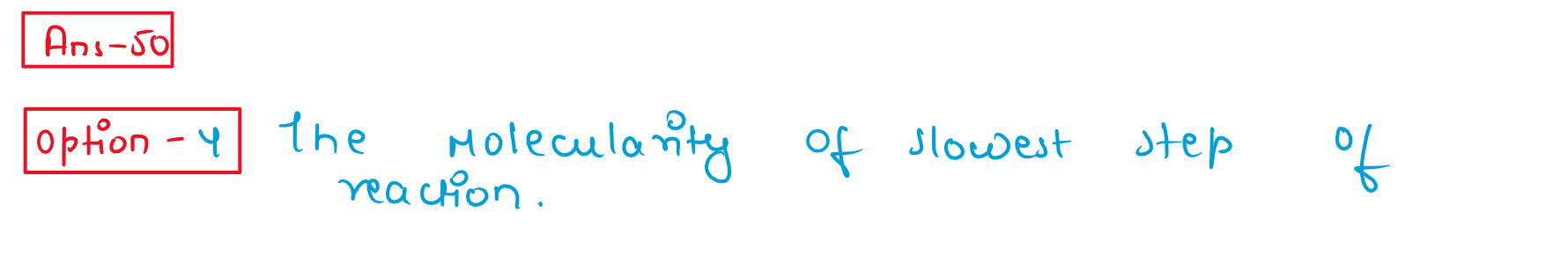




 Free Mock Test Papers
Free Mock Test Papers